A history of pioneering trusted medical solutions to improve the lives we touch

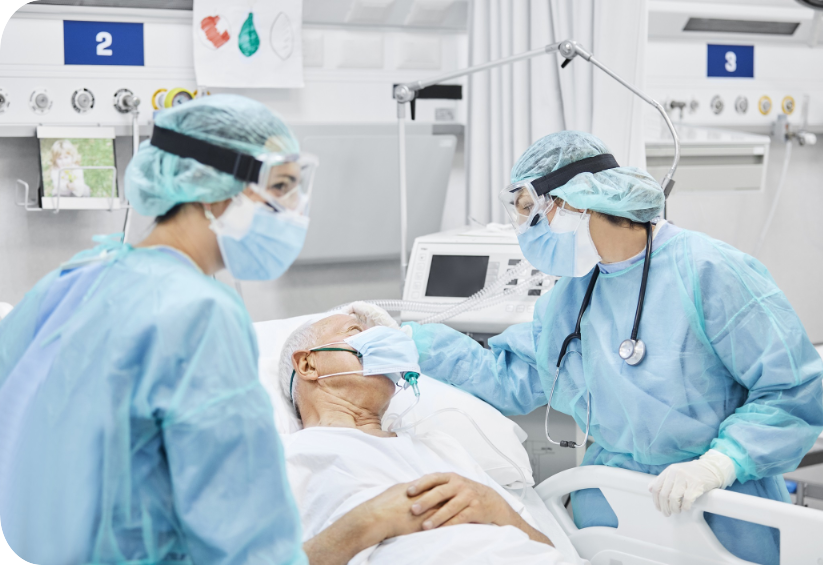
We make fecal management our priority, so it doesn’t have to be yours.
Fecal incontinence – a serious threat to patients
Up to 30% of patients admitted to the ICU experience fecal incontinence1 (FI) during their hospital stay. A serious threat to patients, FI also poses a significant challenge to hospitals to tackle. Especially, FI leads to:

We make fecal management our priority, so it doesn’t have to be yours.

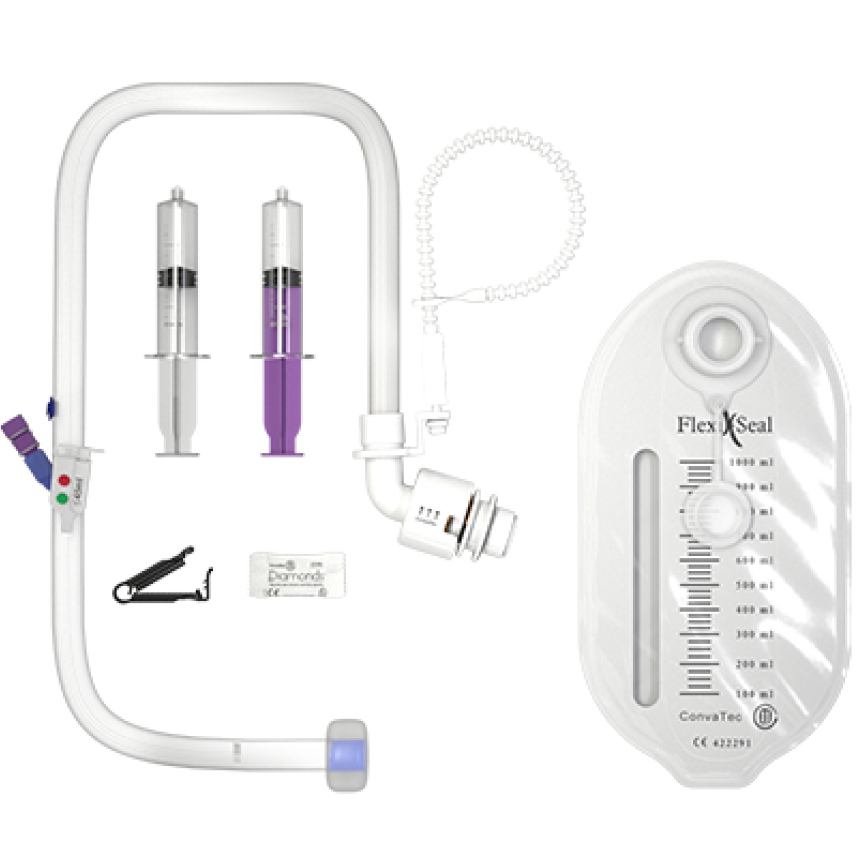
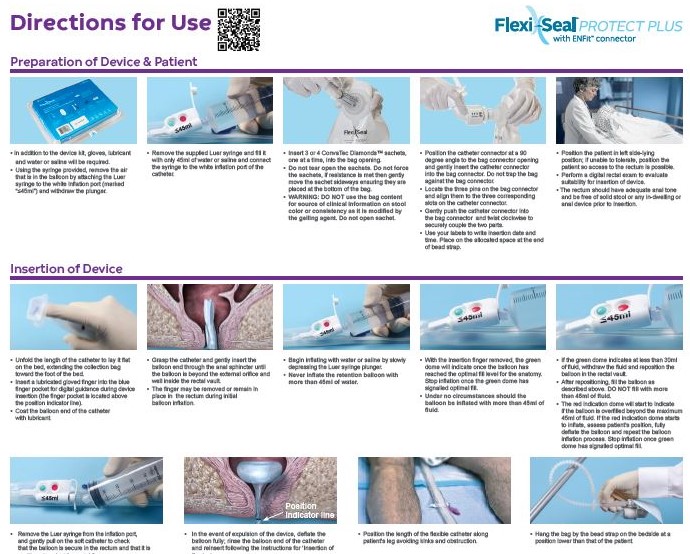
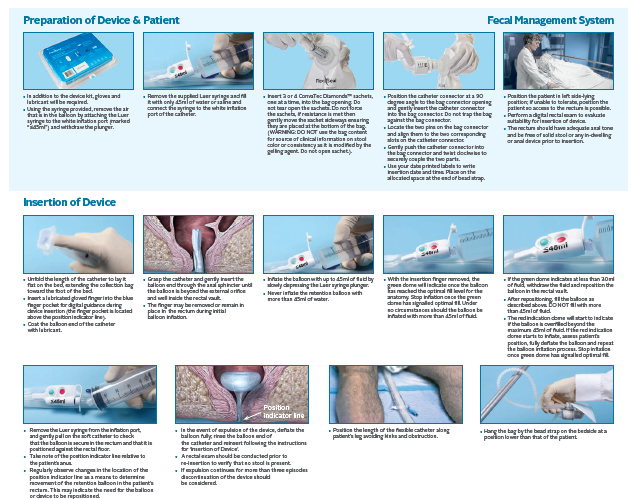
Flexi-Seal™ PROTECT PLUS Fecal Management System is an indwelling fecal management catheter intended for use to manage fecal incontinence through the collection of liquid to semi-liquid stool and to provide access to administer medications. The device is intended for use in adult patients.
The new Flexi-Seal™ PROTECT PLUS is designed with understanding to provide a safe, effective and dignifying solution to fecal management.
Flexi-Seal™ which is designed to help improve outcomes of patients suffering from FI has been shown to reduce complications and costs associated with FI in the hospital setting2.
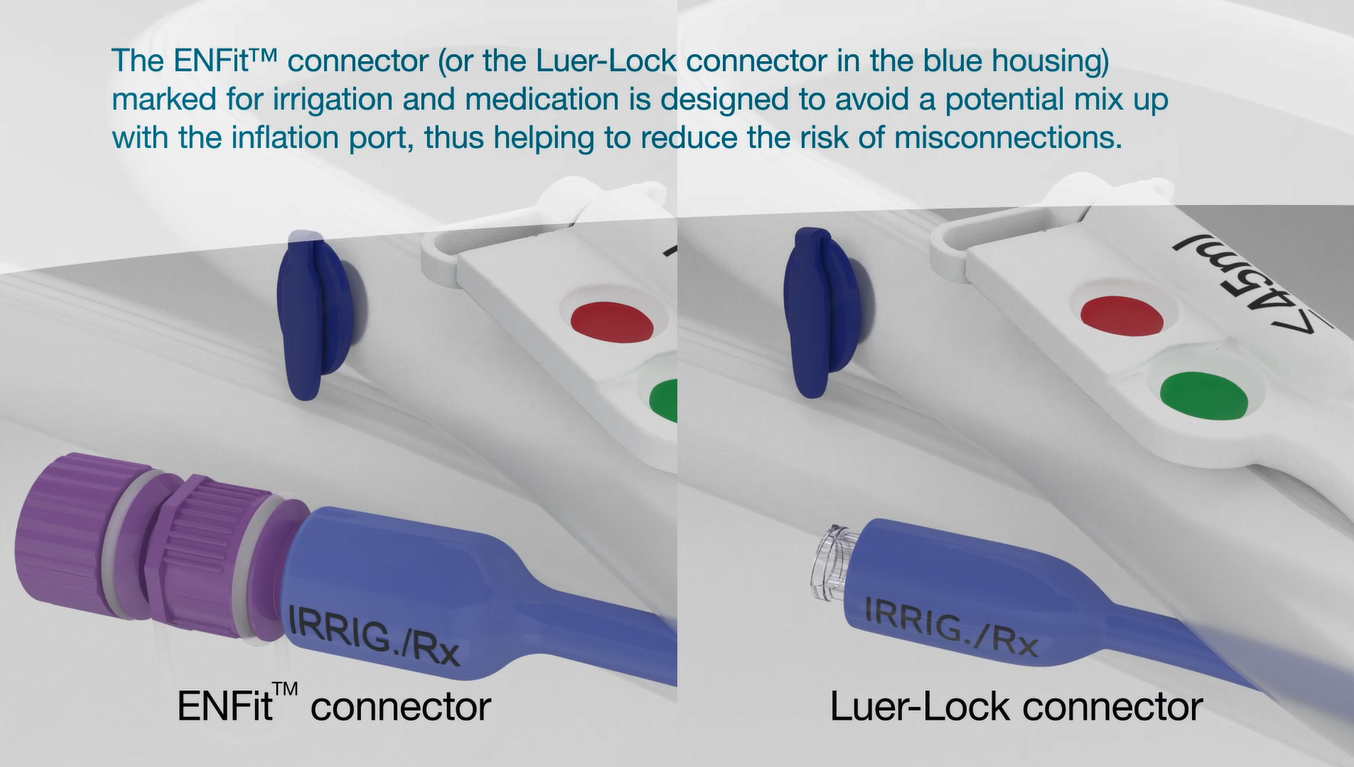
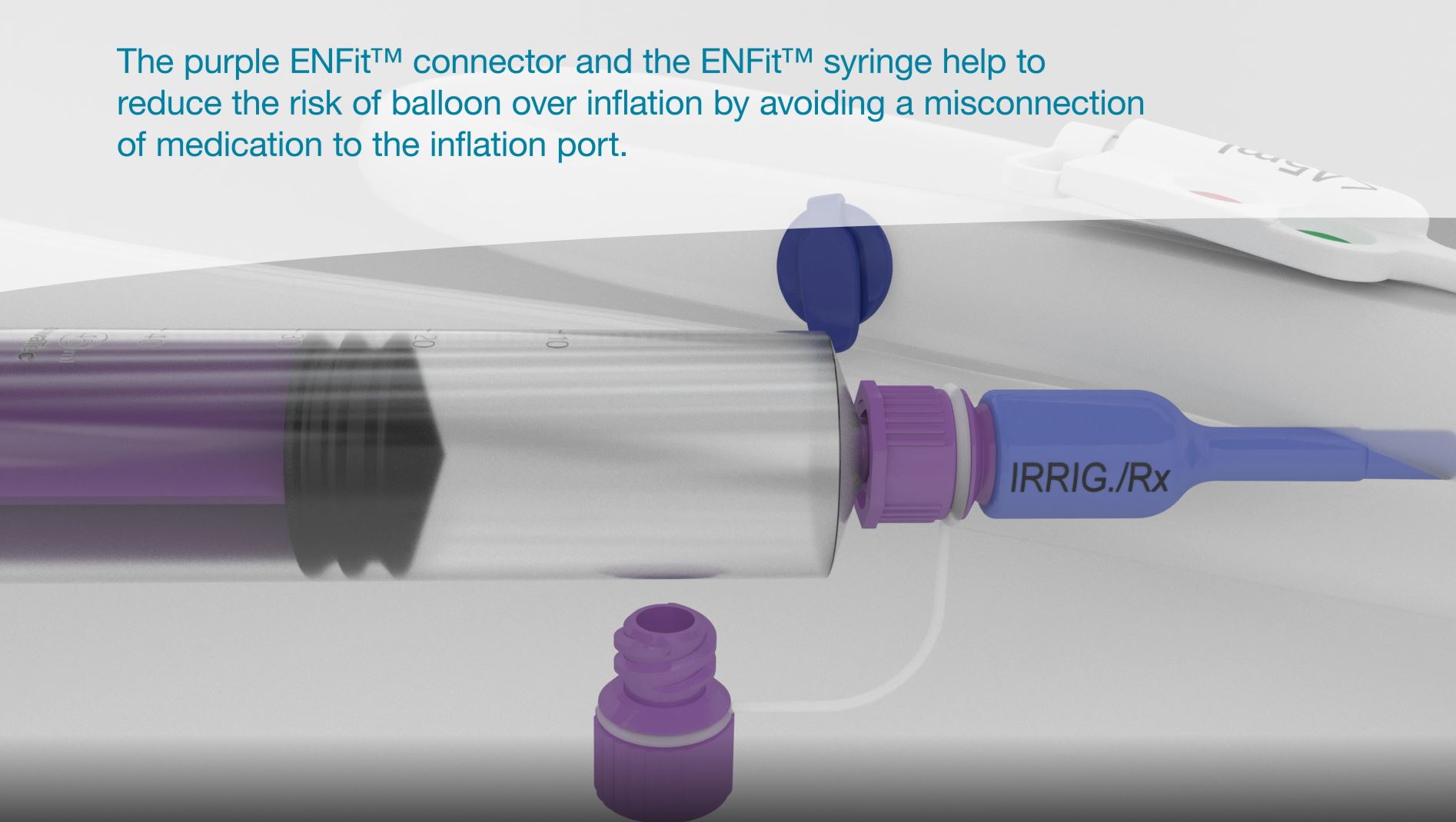
Medication/irrigation port - ENFit™ or Luer connection
Delivers medication effectively via a secondary route. Available with ENFit™ or Luer connector.
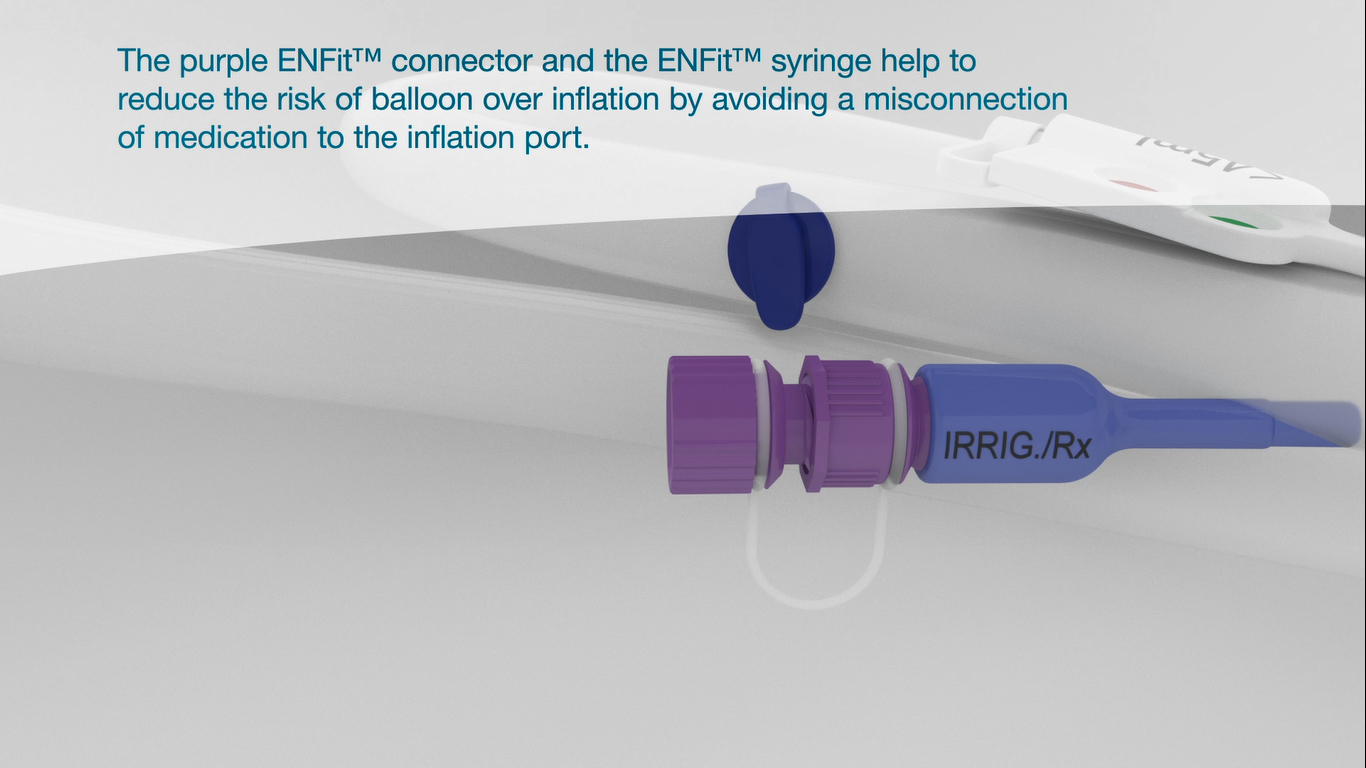
The ENFit™ connector (or the Luer connector in the blue housing) helps to reduce the risk of balloon over inflation by avoiding misconnection of medication to the inflation port.
One-way valve design to prevent leaks or back flow during medication administration or irrigation.
Increased efficiency for Health Care Professionals by administering medication rectally.
Colored sampling port
Designed to facilitate stool sampling. The blue sample port includes a cap and can easily be identified.
It allows for safe and easy stool collection helping to minimize the risk of fecal exposure.
The colored sample port is designed to simplify work routine and reduce nurse effort.
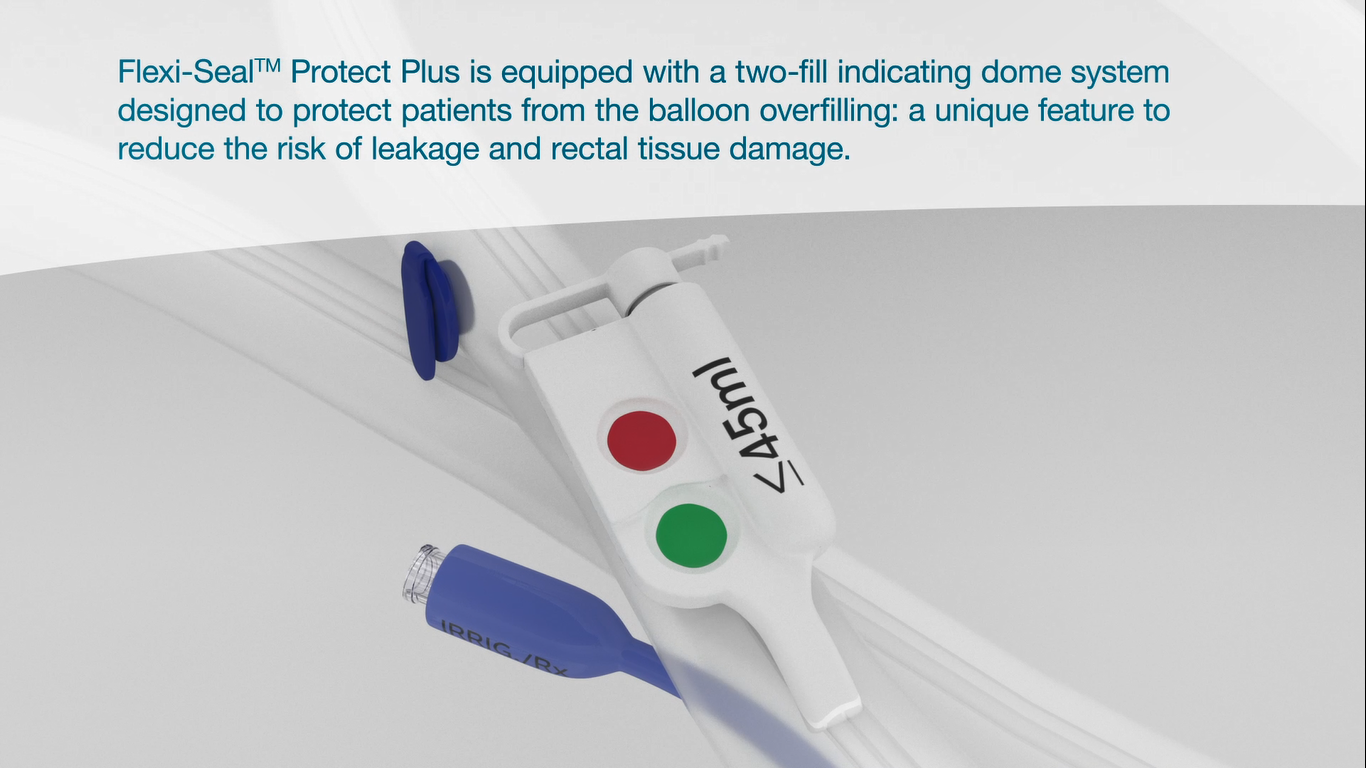
Patient specific inflation indicator / Over inflation warning system
The green dome indicates once the balloon has reached the patient’s individual fill volume.
75% of patients require 40ml or less fill volume for ideal fit and reduced leakage7.
Liquid stool in contact with skin is a major risk driver for pressure ulcers with each estimated to cost 40,000 USD8.
The red dome indicates if the balloon is over-filled. Over inflation beyond the optimal fit for individual patient may lead to complications.
Over inflation can lead to severe rectal tissue damage that requires prolonged treatment & hospitalization9.
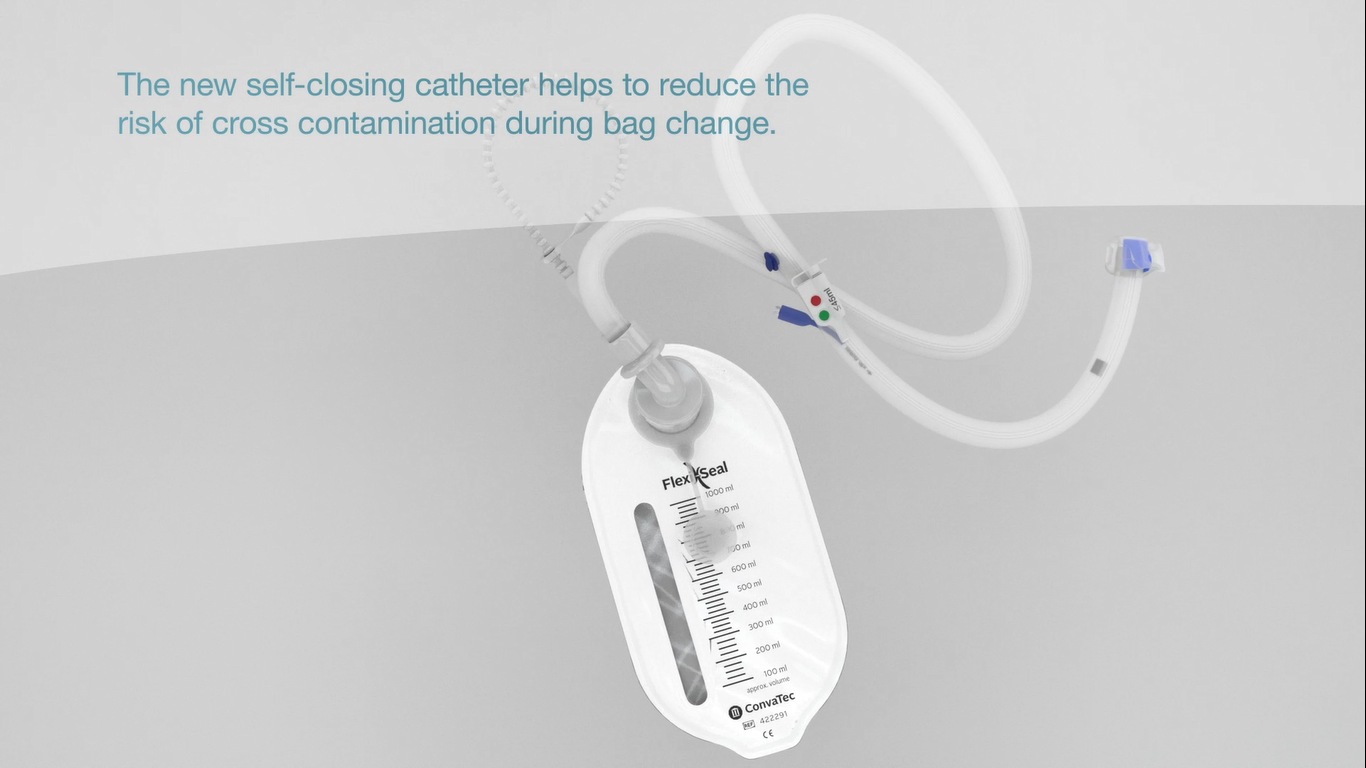
Self-closing catheter
Designed to reduce the risk of leakage and cross-contamination during bag change.
The self-closing catheter and the Privacy™ collection bag create a closed system that helps to reduce the risk of C. difficile contamination.
For increased caregiver confidence.
Helps to reduce the risk of CDI with a mean cost per hospital stay in the USA of $24,40010.
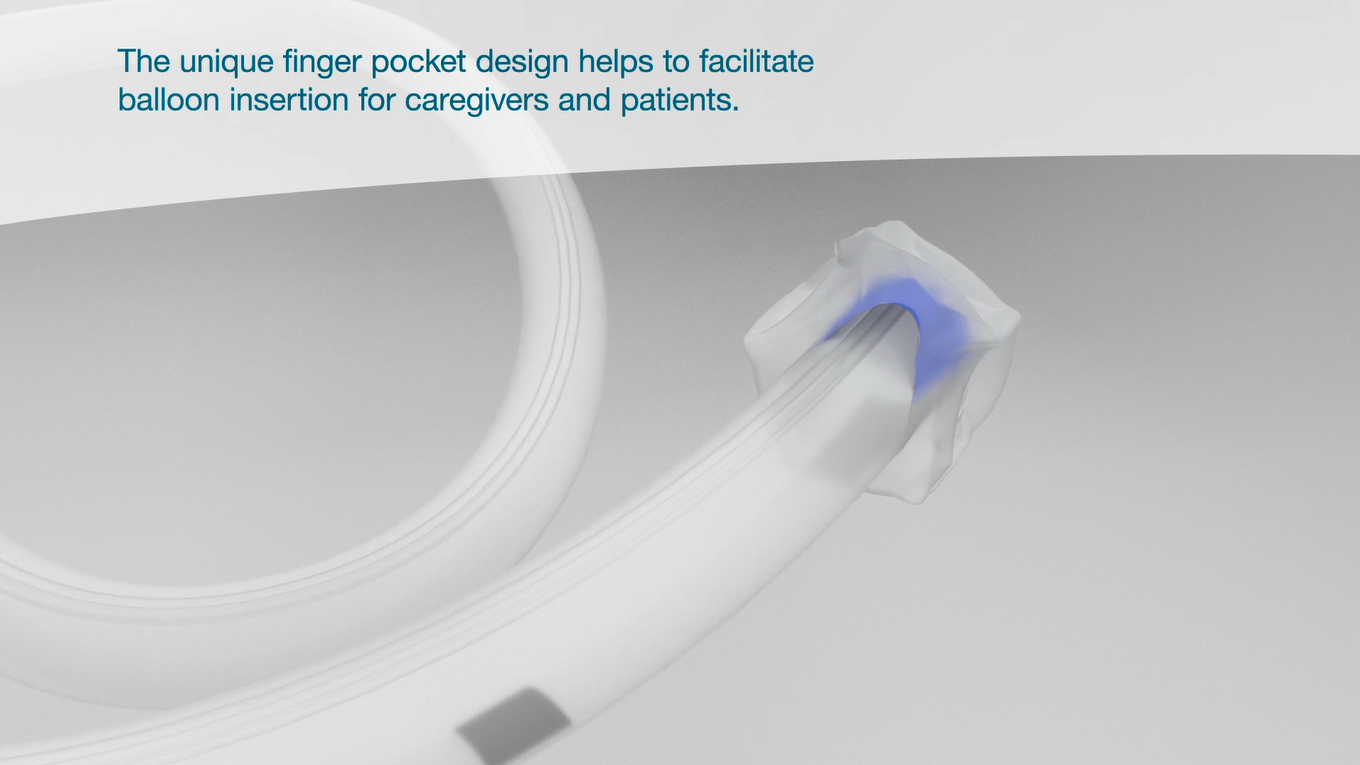
Blue finger pocket
Designed to allow easy and accurate placement of the soft silicone retention balloon.
The unique finger pocket design helps to facilitate balloon insertion for caregivers and to protect patients.
Helps to apply the catheter faster which saves time and worries.
Soft Balloon
Soft, flexible retention balloon conforms to the sphincter anatomy. It creates an effective seal to minimize leakage and is designed to minimize the chance of tissue necrosis.
Compared with competitor products, Flexi-Seal™ PROTECT PLUS is designed to help reduce cross-contamination risk by avoiding splatter upon removal11.
Helps to reduce the risk of CDI with a mean cost per hospital stay in the USA of $24,400.1010.
Zeolite™ silicone catheter
Odor barrier runs the full length of the catheter, providing an end-to-end odor barrier12.
Improves patient and caregiver experience and increases quality of life by reducing odor in the treatment area.
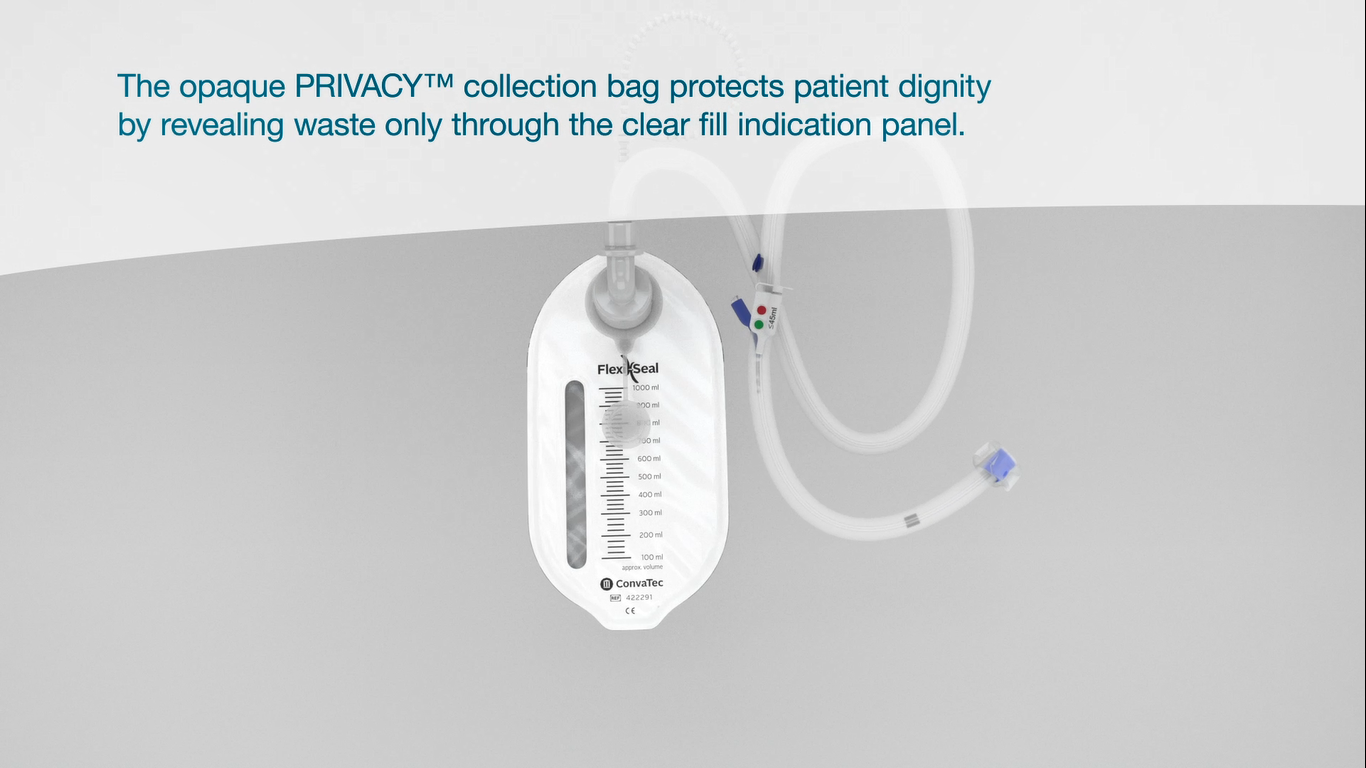
Privacy™ collection bag with active charcoal filter
Including 4 ConvaTec Diamonds™ gelling and odor control sachets.
Designed to contain and lock in odor. Opaque bag only reveals waste through the clear fill indication panel to protect patient dignity.
Minimizes the spread of C. difficile spores by releasing air through the active charcoal filter. Captures 6 times more odor13 than charcoal-filtered collection bags. This filter also eliminates the need to manually release the excess gas from the collection bag14.
Helps to reduce the risk of CDI with a mean cost per hospital stay in the USA of $24,40010.
Gelling and odor control sachets
ConvaTec Diamonds™ are gelling and odor control sachets with active charcoal to reduce the risk of spillage and odor effectively.
For insertion into the bag during the preparation of the device.
4 ConvaTec Diamonds™ gelling and odor control sachets are pre-packed in Flexi-Seal™ Protect Plus kit & the replacement bags.
Medication/Cinch clamp
The cinch clamp is intended for clamping the catheter during administration of medication. It is applied on the catheter at the black indicator line.
During the time of application, the cinch clamp prevents medication from back flow, so it can dwell in the rectum for the required amount of time.
Increased efficiency for Health Care Professionals by administering medication rectally.
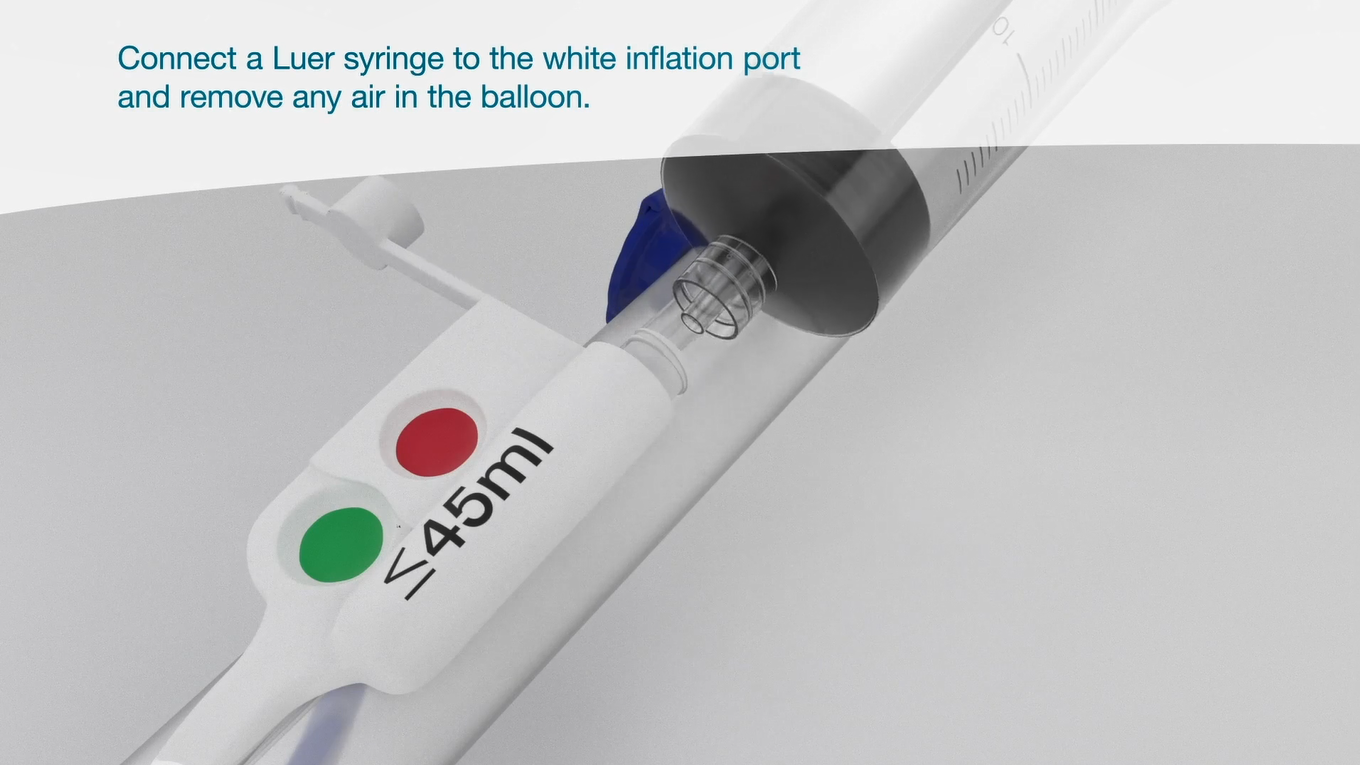
Luer syringe
The Luer syringe is intended for inflation of the balloon through the white inflation port (marked “≤45ml”).
It contains graduation marks up to 45 ml to indicate the maximum fill volume, thus helps caregivers to prevent over inflation.
Over inflation beyond the optimal fit for individual patient may lead to complications.
Over inflation can lead to severe rectal tissue damage that requires prolonged treatment & hospitalization.9
ENFit™ syringe
(only available in Flexi-Seal™ Protect Plus ENFit™ versions)
The ENFit™ syringe is used for irrigation of the device and administration of medication through the irrigation/medication port housing (marked “IRRIG./Rx”).
The ENFit™ tip is designed to avoid any potential mix up with the inflation port, thus helping to reduce the risk of misconnections.
Increased efficiency for Health Care Professionals by administering medication rectally.
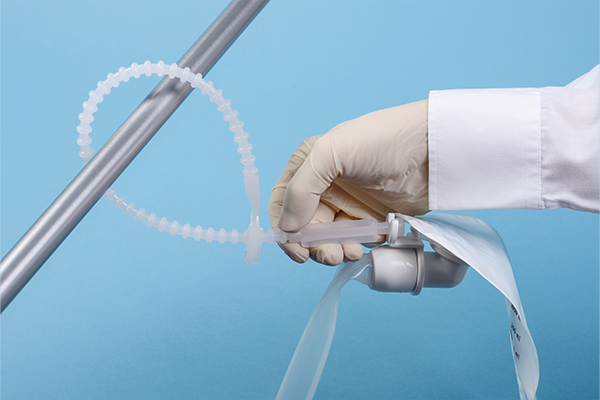
Bead strap
Designed to hang the collection bag on the bedside in a suitable and convenient position.
The bead strap can be locked at the desired length. Its swivelling functionality allows for easy movement of the bag.
How does Flexi-Seal™ PROTECT PLUS aid fecal management?
Essential Resources
Select documents to download, or click to open PDF.

Reach out and learn more
How we can best support you to achieve improved patient outcomes together
- Bayón García, Cristina & Binks, Rachel & De Luca, Enrico & Dierkes, Christine & Franci, Andrea & Gallart, Elisabet & Niederalt, Georg & Wyncoll, Duncan. (2012). Prevalence, management and clinical challenges associated with acute faecal incontinence in the ICU and critical care settings: The FIRSTTM cross-sectional descriptive survey. Intensive & critical care nursing : the official journal of the British Association of Critical Care Nurses. 28. 242-50.
- Langill M, Yan S, Kommala D, et al. A Budget Impact Analysis Comparing use of a Modern Fecal Management System to Traditional Fecal Management Methods in Two Canadian Hospitals. Ostomy Wound Management 2012; 58(12)25-33.
- CDC FAQ on C. difficile. Available at: http://www.cdc.gov/cdiff/index.html, Accessed 25th of October 2019
- Lessa FC, Mu Y, Bamberg WM, Beldavs ZG, Dumyati GK, Dunn JR, et al. Burden of Clostridium difficile Infection in the United States. New England Journal of Medicine. 2015;372(9):825–34.
- Schroeder MS. Clostridium difficile-associated diarrhea. Am Fam Physician. 2005;71(5);921-928.
- Zhang S, Palazuelos-Munoz S, Balsells E M, Nair H, Kyaw M H. Cost of hospital management of Clostridium difficile infection in United States—a metaanalysis and modelling study. BMC Infect Dis. 2016; 16(1): 447.
- Optimizing Fecal Containment Using Individualized Balloon Volumes; Catherine T. Milne APRN, MSN, BC-ANP, CWOCN1; Ann Durnal RN, BSN, CWOCN2; Mary Webb, RN, BSN, MA, CIC3, 1Connecticut Clinical Nursing Associates, LLC, Bristol, Connecticut; 2Ascension Carondelet St Mary’s Hospital, Tucson, Arizona;3San Mateo Medical Center, San Mateo, California
- Jackson M, McKenney T, Drumm J, Merrick B, LeMaster T, VanGilder C. Pressure ulcer prevention in high-risk postoperative cardiovascular patients. Crit Care Nurse. 2011;31(4):44–53
- MAUDE FDA Data Analysis – Accessed during January 2019 – Data on file, ConvaTec.
- Lucado J, Gould C, Elixhauser A. Clostridium difficile infections (CDI) in hospital stays, 2009. HCUP Statistical Brief 124. Rockville, MD: Agency for Healthcare Research and Quality. Available at: http://www.hcup-us.ahrq.gov/reports/ statbriefs/sb124.pdf.
- Metcalf et al. Contamination Risk During Fecal Management Device Removal: An In vitro, Simulated Clinical Use Study. Wound Manage Prev 2019; 65(3): 30–37.
- Odor Barrier Testing. 130124-001. Data on file, ConvaTec Inc.
- Flexi-Seal® Privacy Bag Filter Evaluation. 121412-001. Data on file, ConvaTec Inc.
- Minimizing the spread of C. difficile spores from the release of gas. February 19, 2013. Data on file, ConvaTec Inc.
- García B, Binks R, De Luca E, Dierkes C, Franci A, Gallart E, Niederalt G, Wyncoll D, Vaes P, Soderquist B, Gibot S. Expert recommendations for managing acute fecal incontinence with diarrhea in the intensive care unit. J Intensive Care Soc. 2013;14(Suppl 2):1-9.
- Jones S, Towers V, Welsby S, et al. Clostridium difficile Containment Properties of a Fecal Management System: An In Vitro Investigation. Ostomy Wound Management 2011;57(10):38–49.